Our work combines computational frameworks with wet lab techniques to design and optimize metabolic networks for diverse applications.
Tools for Metabolic Engineering
Machine Learning Frameworks
We research machine learning approaches that address challenges in protein function prediction. Kinetic parameters are essential for understanding enzyme functionality within metabolic networks and their regulation by various compounds. We developed CPI-Pred, a deep learning model to predict kinetic parameters based on compound-protein interactions. This model integrates compound representations generated by a message-passing neural network with enzyme representations derived from protein language models, allowing for accurate predictions across diverse enzyme-compound pairs.
Additionally, we address the challenge of combinatorial growth in potential reaction networks when searching for novel biochemical pathways. We created Anneal Path, a computational tool for the efficient enumeration of branched pathways. It utilizes a hypergraph-based model to represent multimolecular reactions and enzyme promiscuity on multiple substrates. Our approach allows for a more comprehensive exploration of the chemical space, identifying a larger set of branched pathways compared to existing methods.
Datasets and Databases
We compiled an extensive dataset of enzyme kinetic parameters, encompassing metrics including the Michaelis-Menten constant (KM), enzyme turnover number (kcat), catalytic efficiency (kcat/KM), and inhibition constant (KI). This dataset serves as valuable benchmark for the scientific community, supporting further development of machine learning models, such as CPI Pred.
Furthermore, we developed KinMod, a database that consolidates over 2 million data points on metabolic regulation across 9,814 organisms. By integrating multi-omics data, KinMod facilitates machine learning approaches, such as kinetic parameter estimation and metabolic model construction, enabling researchers to explore regulatory interactions and identify gaps in experimental measurements.
Genome-Scale Models
We develop genome-scale metabolic models (GEMs), a powerful systems biology approach to cellular engineering, offering predictive frameworks that encompass entire metabolic networks. By representing the complete metabolic potential of an organism, these models support hypothesis-driven engineering strategies that would be difficult to formulate through traditional reductionist approaches.
Biological systems operate across remarkable ranges of time scales—from enzymatic reactions occurring in seconds to evolutionary processes spanning years—and across multiple length scales from nanometers within cells to meters in environmental microbial communities. Our research develops GEMs that can capture the complexity of these systems by integrating kinetic data, enabling a more accurate representation of their dynamic and multi-scale nature.
Biosensor Development
We design and optimize transcription-factor based biosensors to provide accurate detection and relative quantification of various biochemicals. Utilizing advanced rational engineering approaches, including structure-guided engineering, computational molecular docking, and fluorescence activated cell sorting, we have engineered biosensors for increased specificity of adipic acid, an important industrial chemical used in nylon production. Combining these rational strategies with novel approaches, such as chimeric transcription factor libraries and continuous in vivo random mutagenesis, we aim to transform biosensor development.
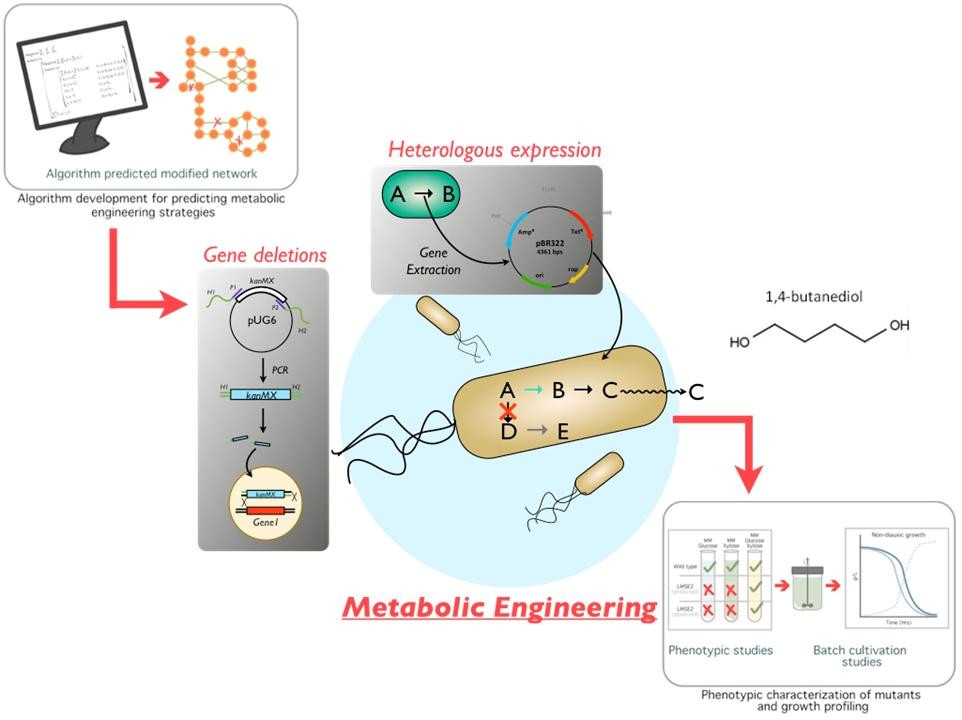
Applied Metabolic Engineering
Chemical and Fuel Production
We work at the intersection of microbiology, biochemistry, and systems biology to develop bioprocesses with significant environmental and economic benefits. Our goal is to create microbial platforms that convert waste streams into valuable products for industrial applications.
We optimize microbial feedstock utilization for bioprocess engineering. Potato starch and ethylene glycol (EG) are promising renewable feedstock candidates as they can easily be sourced from PET plastic depolymerisation and farm waste. Our lab has engineered S. cerevisiae to efficiently breakdown potato starch via the directed evolution of heterologous amylases and rational strain design. Moreover, using adaptive laboratory evolution, we have isolated an E. coli strain that can consume EG as a sole carbon source. These microbial strains will be integrated into future bio-manufacturing processes and facilitate the utilization of renewable feedstocks within industry.
Moreover, our research harnesses the unique capabilities of chain elongating bacteria. These bacteria naturally produce medium-chain fatty acids (C6-C8), valuable platform chemicals that can serve a wide range of industrial applications. We are exploring their potential to synthesize longer-chain fatty acids (C9-C12) and diverse oleochemicals, such as fatty alcohols, dicarboxylic acids, diols, and methyl ketones. We have found critical trade-offs between the carbon length and class of target products and the growth rates of these bacteria, offering insights into resource allocation during biosynthesis. Additionally, we are developing tools for the genetic manipulation of chain elongating bacteria, laying the groundwork for enhanced bioconversion capabilities.
Human Health
Through metabolic engineering, we explore the intricate connection between human health and microbial metabolism. Our research focuses on the gut microbiome, a diverse ecosystem of around 1,000 microbial species that contributes nearly 2 kg of biomass and over a million genes to human biology. We engineer native and probiotic bacterial communities for therapeutic and diagnostic applications, targeting conditions such as obesity, diabetes, and inflammatory bowel disease.
Environmental Biotechnology
We develop advanced biomining technologies to remediate acid mine drainage (AMD) and recover valuable metals from mining tailings. Our lab recently isolated the dominant acidophile Acidithiobacillus ferridurans JAGS, an iron-sulfur oxidizing bacterium that uses chemical energy from iron oxidation for metabolism. This acidophile naturally facilitates nickel bioleaching, offering a more environmentally sustainable alternative to conventional mining technologies. Through genomic and metabolomic analyses, we’re creating a knowledge foundation CRISPR-mediated engineering to develop an enhanced microbe with improved biomining efficiency; making the process more robust, sustainable, and effective for nickel recovery and mine site remediation.
Furthermore, our research addresses sustainability challenges in the cultivated meat industry by developing affordable alternatives to recombinant transferrin, a costly component in serum-free media. We employ advanced techniques including genome mining, AlphaFold2 structural modeling, and affinity purification mass spectrometry, to identify and characterize plant-based proteins from legumes with transferrin-like properties. Our promising preliminary results indicate structural similarities that could support iron-binding and cellular functions. This approach could dramatically reduce media costs, enhancing scalability and sustainability in alternative protein production.
